The 8 Wastes of Lean
Do you, in your research administration office, require signatures on grant proposals or forms that aren't technically required? You may have "waste" in your Research Admin processes.

Don't worry, we all do.
One of the aims of Lean Research Administration is to reduce waste through process improvement.
Research Administration is to reduce waste through process improvement.
Here's how to identify and remember the eight types of waste that exist in processes. But really what is waste? Sounds...subjective. You can take the subjectiveness out of waste reduction by using The 8 Wastes (aka DOWNTIME).
Acronyms are not unfamiliar to research administrators or anyone working in research.

DOWNTIME is one you need to know.

Defects are the same as errors.

Overproduction. Research administrators love processing, sometimes too much. Ever pulled date for a report that no one looks at? That's overproduction.
Waiting is the most common waste. Customers (PIs) wait, sponsors wait, we wait. Everyone is waiting. Can we make processes with less waiting waste? That's a great place to start if you are new to lean research administration.

Non utilized talent. If you aren't using folks to their full potential, they will leave. Full stop.
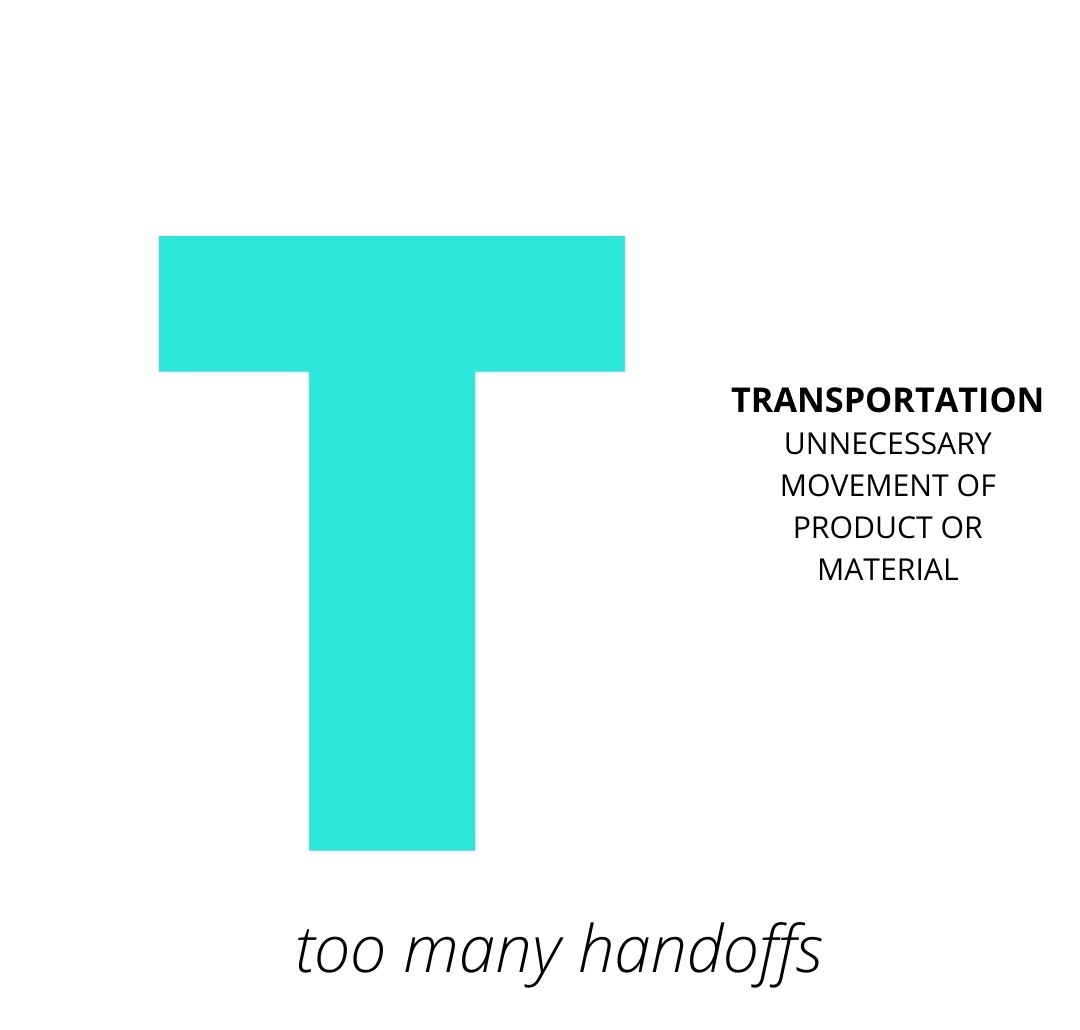
Transportation - an approval passes through HOW MANY HANDS?
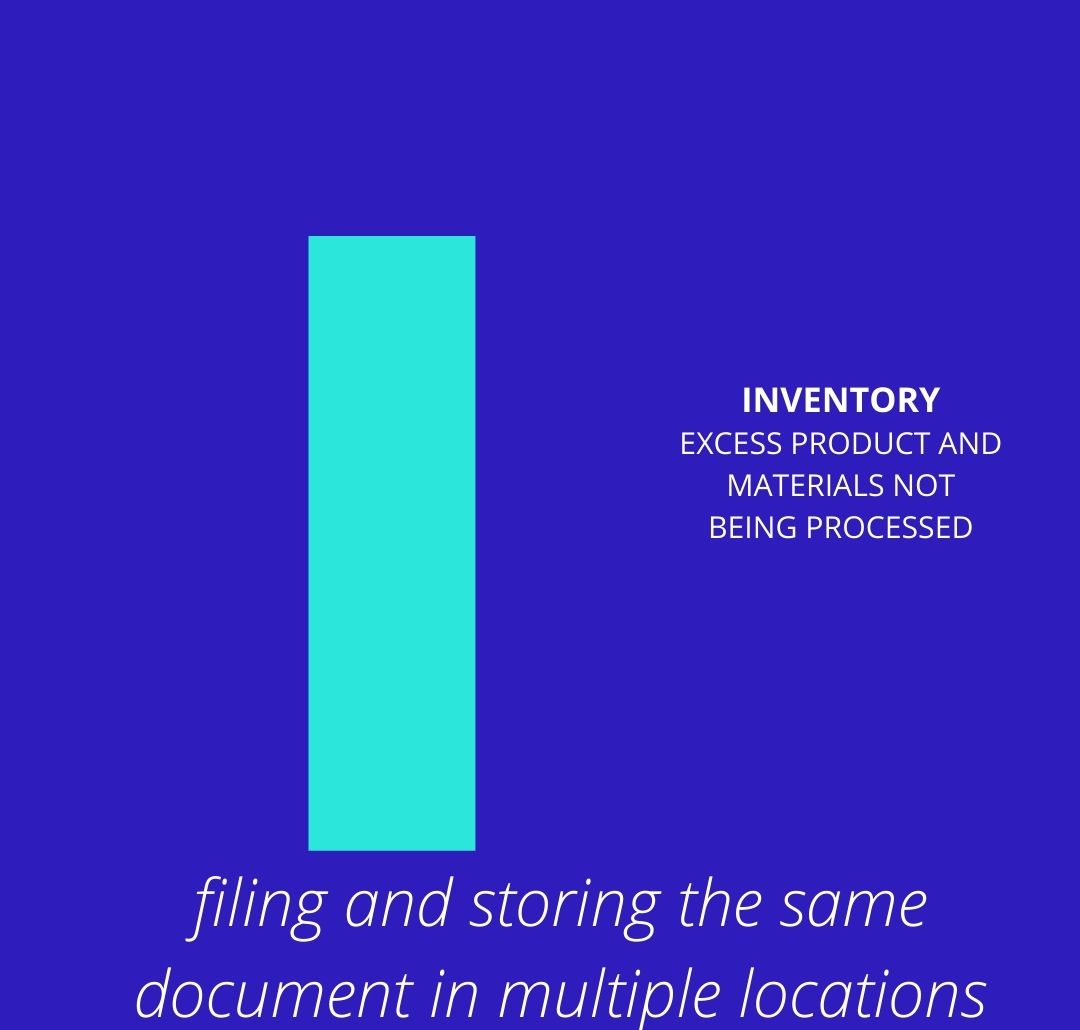
Inventory can be putting documents in multiple areas unecessarily, but it might also be a research team ordering too many kits, that then expire. Or too little. Sometimes inventory is tough to manage in clinical research, but that's why we lean research and research administration!

Are administrators looking in six different systems in order to complete a task? That's too much motion. Shoot for five!!
Research Administrators are exceptionally good at creating controls. The balance is between having a tight system of control without extra or excessing processing. This can be requiring signatures outside of those required.
Where is there waste in research and clinical research administration processes?

Don't worry, we all do.
One of the aims of Lean
 Research Administration is to reduce waste through process improvement.
Research Administration is to reduce waste through process improvement.Here's how to identify and remember the eight types of waste that exist in processes. But really what is waste? Sounds...subjective. You can take the subjectiveness out of waste reduction by using The 8 Wastes (aka DOWNTIME).
Acronyms are not unfamiliar to research administrators or anyone working in research.

DOWNTIME is one you need to know.

Defects are the same as errors.

Overproduction. Research administrators love processing, sometimes too much. Ever pulled date for a report that no one looks at? That's overproduction.

Waiting is the most common waste. Customers (PIs) wait, sponsors wait, we wait. Everyone is waiting. Can we make processes with less waiting waste? That's a great place to start if you are new to lean research administration.

Non utilized talent. If you aren't using folks to their full potential, they will leave. Full stop.

Transportation - an approval passes through HOW MANY HANDS?

Inventory can be putting documents in multiple areas unecessarily, but it might also be a research team ordering too many kits, that then expire. Or too little. Sometimes inventory is tough to manage in clinical research, but that's why we lean research and research administration!

Are administrators looking in six different systems in order to complete a task? That's too much motion. Shoot for five!!

Research Administrators are exceptionally good at creating controls. The balance is between having a tight system of control without extra or excessing processing. This can be requiring signatures outside of those required.
Where is there waste in research and clinical research administration processes?